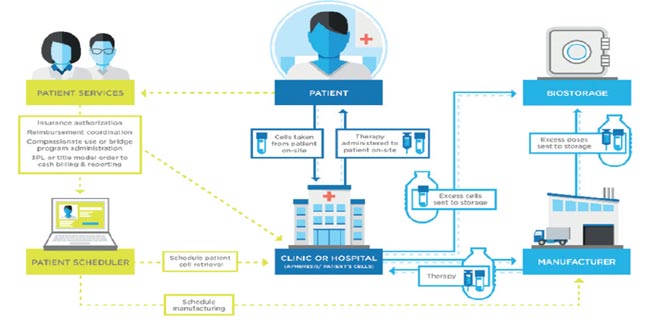
Applying validation standards to bio/pharmaceutical logistics, the science of transporting bio/analytical, clinical and manufactured product, is an important and essential challenge as global demand for biotechnology continues to rise.
While proposals for basic standards have been brought forward, consensus throughout the industry regarding best practices or even the importance of logistics validation has yet to be reached (Suhong Li, 2005). Reasons for differences are as complex as they are justifiable, including but not limited to differences in value and the formulation/stability of the material being shipped, varying availability of logistics infrastructure in the market of interest and cost of validation and implementation procedures (A. Coustasse, 2016). However, logistics networks are becoming increasingly complex as ever more environmentally astute technology is developed and demand for biologics and pharmaceuticals from emerging markets such as Asia and Africa continues to rise (Fig.1) (Pharmaceutical Commerce, 2017), and next generation cell and gene therapies are brought to the market. Additionally, regulatory changes are necessitating the development of organizational infrastructure to record product condition and authenticity data to ensure that the quality and safety of a given product is not compromised while in transit (Health Service Authority, 2015).
As often happens within industries, emerging requirements for increased, improved or imposed operational control/regulation coopts familiar terminology and/or systems but applies it under new premises to address different issues altogether. This leads to confusion, and in fact friction within the industry, in this case bio/pharmaceutical development, as systems and processes integrate across, for example, clinical development and commercial logistics. Both parties use the term validation towards the same basic goal (establish a baseline to which data be analyzed/compared) but apply it in very different ways. Understanding these differences is vital to improving efficiency, cooperation and most importantly, sample and product quality throughout the bio/pharmaceutical development process.
Bio/pharmaceutical standards for validation are well established, the key aspects of which are reproducibility, precision and accuracy. While there are some significant differences in standards and execution between differing departments within the industry, namely bioanalytical, clinical and manufacturing, the basic meaning and purpose of validation remain very similar, specifically ensuring that the data or product from any given process will be accurate, influence by internal confounding factors is not only understood but mitigated to the best possible degree, and most importantly, the data/product is reproducible within the validated system (Okhamafe, 2002).
For example, changes in lots of reagents for bioanalytical analysis requires a complex lot bridging process that is designed to ensure that the performance characteristics of an assay are not being confounded by changing the reagents themselves (Dimeglio, 2012). These processes ensure that all samples and product are obtained and analyzed under a very specific set of conditions so that all resulting data is comparable as like for like.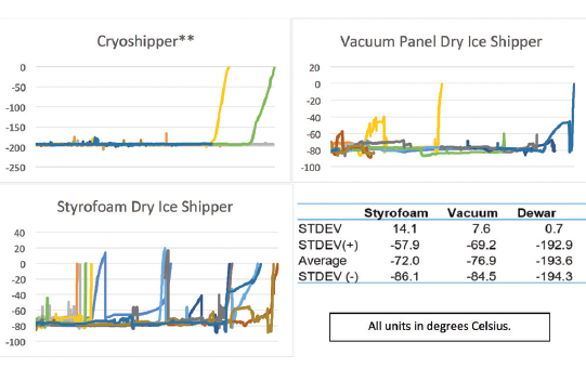 Logistics validation is designed to ensure that risk is mitigated and the system is responsive to external confounding factors, most of which are beyond control. This risk mitigation process works to ensure that the condition and quality of the payload is maintained and a like for like comparison between payloads remains viable while recognizing that due to the constantly changing global logistics environment, like for like comparisons between the events of individual shipments is impossible (i.e. customs delays, mishandling, weather, etc.). Therefore, the purpose of logistics validation is better described as a proactive baseline for understanding and mitigating the risk to a product while in transit (Fig. 2). This is done by validating several key factors, namely packaging, logistics and data management, collectively referred to as “Intelligent Logistics” (Cryoport, 2016).
Logistics validation is designed to ensure that risk is mitigated and the system is responsive to external confounding factors, most of which are beyond control. This risk mitigation process works to ensure that the condition and quality of the payload is maintained and a like for like comparison between payloads remains viable while recognizing that due to the constantly changing global logistics environment, like for like comparisons between the events of individual shipments is impossible (i.e. customs delays, mishandling, weather, etc.). Therefore, the purpose of logistics validation is better described as a proactive baseline for understanding and mitigating the risk to a product while in transit (Fig. 2). This is done by validating several key factors, namely packaging, logistics and data management, collectively referred to as “Intelligent Logistics” (Cryoport, 2016).
Regarding packaging, it is critical to have validated manufacturing and testing methods to ensure both design and manufacturing standards are met to maintain quality across a fleet of shippers. One useful methodology of validation for temperature controlled packaging is the ISTA-7E testing program that validates thermal packaging by testing a shipper’s ability to withstand real world shipping and receiving stressors while maintaining shipper performance. These stressors include durability, temperature stability of the payload area under various adverse environmental and handling conditions, vibration testing, etc. (ISTA, 2017). Collectively, these tests provide a baseline as to the quality of design and expected performance of a shipper model.
However, herein lies a key difference in understanding how validation is used by biopharmaceutical science and logistics, namely, the degree of scalability. In biopharmaceutical science, there is the need to develop and validate one assay to work with one specific lot of reagents, essentially making the assay single purpose if not single use. Therefore, it is possible and in fact required, to validate every assay to industry (GLP/GMP) standards.
While validating individual reusable shippers to ensure like for like comparability may be possible on a very small scale, validating every unit of either disposable or reusable packaging would not only be incredibly time consuming and impractical, but prohibitively expensive especially as demand for a given product scales into the need for thousands if not millions of units under GDP standards (Health Service Authority, 2015).
It is important to note, however, that inherent to any manufacturing process there are bound to be differences between the individual shippers. Understanding those differences is critical to ensuring that a packaging system will meet the needs of a particular mission with the least risk to the payload possible.
Additionally, packaging systems are subject to wear and tear while in transit. In fact, a recent logistics study conducted by Cryoport, Kansas City Analytical Services and Heat Biologics found that out of 33 shippers tested, only one was not mishandled and each individual shipper was mishandled between 15 and 25% of the total time in transit (Fig. 3). Therefore, the characteristics and performance of any packaging system will change over time and even within a single transit event as stress events accumulate.
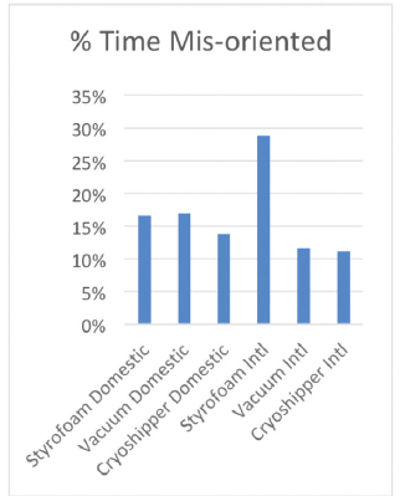 Therefore, while from a feasibility standpoint, best practice is to apply validation data from a statistically significant number of representative shippers across a model packaging system, it is essential that each unit be individually qualified, and in the case of reusable packaging, requalified regularly, so the performance characteristics of that individual packaging system and how it changes over time is well established. In this way, operational control can be maintained and risk to any given payload can be mitigated to the greatest extent possible. Moreover, it is advantageous for companies to utilize in field data to dynamically understand the risk of in-transit events on the performance of the packaging system and its impact on the commodity being shipped in near real time, providing the ability to intercede if required.
Therefore, while from a feasibility standpoint, best practice is to apply validation data from a statistically significant number of representative shippers across a model packaging system, it is essential that each unit be individually qualified, and in the case of reusable packaging, requalified regularly, so the performance characteristics of that individual packaging system and how it changes over time is well established. In this way, operational control can be maintained and risk to any given payload can be mitigated to the greatest extent possible. Moreover, it is advantageous for companies to utilize in field data to dynamically understand the risk of in-transit events on the performance of the packaging system and its impact on the commodity being shipped in near real time, providing the ability to intercede if required.
The second and perhaps most difficult validation target for biopharmaceutical logistics is lane validation, or the process of developing a clear, integrated supply chain with predictable and repeatable shipping and receiving performance.
For example, when undertaking carrier selection it is important to recognize that carrier performance differs wildly based on regional expertise and infrastructure. To that end, the importance of redundancy within a supply chain cannot be overstated. It is quickly becoming standard industry practice to pick multiple carriers over a particular lane to mitigate risk in the case of shipping exceptions such as weather, damage to packaging, routing errors, etc. By sending multiple test shipments over a specific lane, statistically significant total time in transit and on time delivery performance data (collectively referred to as lane mapping) is obtained and used not only to successfully establish complex supply chains, but also successfully and proactively identify deviations.
Regulatory and customs expertise is critical to ensuring on time performance as well. The regulatory environment as well as customs and trade practices are constantly changing. It is therefore important to maintain a clear understanding of not only the product classification, but also the customs and regulatory requirements of the country in which a clinical trial or commercial market expansion is being conducted. When applied, lane validation affords a measure of predictability within an inherently unpredictable logistics environment.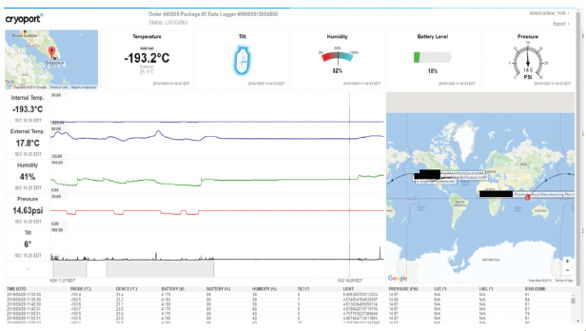 The third piece to logistics validation is one in which both biopharmaceutical science and logistics are in complete agreement, validation of data management systems. Data management is arguably the most critical component of “intelligent logistics”, as it provides the data needed to make datadriven choices regarding logistics as well as monitoring payload conditions and carrier performance in near real time (Cryoport, 2017). Most importantly, data integrity is vital for a proactive instead of reactive approach to logistics. Instead of losing visibility on a product while in transit, it is now possible to monitor that product using cellular enabled condition monitoring systems for added security and product quality as well as risk management. It also provides companies with the ability to apply “Quality by Design” concepts within the logistics space to manage and mitigate risk and better understand variability in an un-controlled system. This data needs to be managed in a comparable way to all other data used to make critical bio/pharmaceutical, bio/analytical clinical and commercial development decisions. 21 CFR part 11 compliance for the acquisition, security and management of data systems provides a clear and readily implementable standard for integration of logistics data as an added metric required to determine product quality and logistics data integrity (U.S. Department of Health and Human Services, Food and Drug Administration, 2017).
The third piece to logistics validation is one in which both biopharmaceutical science and logistics are in complete agreement, validation of data management systems. Data management is arguably the most critical component of “intelligent logistics”, as it provides the data needed to make datadriven choices regarding logistics as well as monitoring payload conditions and carrier performance in near real time (Cryoport, 2017). Most importantly, data integrity is vital for a proactive instead of reactive approach to logistics. Instead of losing visibility on a product while in transit, it is now possible to monitor that product using cellular enabled condition monitoring systems for added security and product quality as well as risk management. It also provides companies with the ability to apply “Quality by Design” concepts within the logistics space to manage and mitigate risk and better understand variability in an un-controlled system. This data needs to be managed in a comparable way to all other data used to make critical bio/pharmaceutical, bio/analytical clinical and commercial development decisions. 21 CFR part 11 compliance for the acquisition, security and management of data systems provides a clear and readily implementable standard for integration of logistics data as an added metric required to determine product quality and logistics data integrity (U.S. Department of Health and Human Services, Food and Drug Administration, 2017).
The drivers behind the advent of advanced pharmaceutical logistics and the need for validation, namely risk mitigation and the preservation of increasingly temperature sensitive technologies, which can be valued at multiple millions of dollars in a single shipment, over ever more complex supply chains has driven the cold chain logistics market to over $12.6 billion annually (Pharmaceutical Commerce, 2017). Importantly, emerging technologies such as regenerative medicine will only increase the complexity of the logistics systems that need to be validated, especially as the requirements of the Drug Supply Chain Security act come into force (U.S. Food and Drug Administration, 2017). A meaningful conversation about the application and standards of systems validation between pharmaceutical science and logistics is long overdue, but more important than ever as systems become increasingly integrated. The result of improved understanding will be more efficient pharmaceutical development, improved product quality and patient accessibility to new technology throughout existing and emerging markets alike.
References
- A. Coustasse, e. a. (2016). Could the Pharmaceutical Industry Benefit from Full-Scale Adoption of RadioFrequency Identification (RFID) Technology with New Regulations? Perspectives in Health Information Management, 1b.
- Cryoport. (2016, 11 18). Intelligent Logistics. Retrieved from Cryoport: http://www.cryoport.com/why-cryoport/ intelligent-logistics.
- Cryoport. (2017, November 7). Cryoport. SmartpakII Condition Monitoring System Brochure. Retrieved from Cryoport Resources: http://www.cryoport.com/resources/ smart-pak-ii-condition-monitoring-system-brochure.
- Dimeglio, e. a. (2012, May 12). Lot Bridging Considerations for Immunoassay Kits in Biomarker Studies. Retrieved November 7, 2017, from Bioagilytics Labs: http://files. bioagilityx2.gethifi.com/resources/posters/lot-bridgingconsiderations-for-immunoassay-kits-in-biomarkerstudies/Kit_Lot_Bridging_Poster.pdf
- Health Service Authority. (2015, August). Guidance Notes on Good Distribution Practice. Retrieved from Health Service Authority: http://www.hsa.gov.sg/content/dam/ HSA/HPRG/Manufacturing_Importation_Distribution/ Guidance%20documents%20for%20Industry/GUIDEMQA-013-010.pdf
- ISTA. (2017, November 7). Testing Standard for Thermal Transport Packaging Used in Parcel Delivery System Shipment. Retrieved from ISTA : https://ista.org/ docs/7Eoverview.pdf
- Okhamafe, E. J. (2002, December). An Overview of Pharmaceutical Validation and Process Controls in Drug Developmemt. Tropical Journal of Pharmaceutical Research, 115-122.
- Pharmaceutical Commerce. (2017, March 15). Pharmacuetical Cold Chain Logistics is a $12.6B Global Industry. Retrieved from Pharmacuetical Commerce. com: http://pharmaceuticalcommerce.com/supply-chainlogistics/pharmaceutical-cold-chain-logistics-is-a-12-6- billion-global-industry/
- Suhong Li, e. a. (2005). Development and validation of a measurement instrument for studying supply chain management practices. Journal of Operations Management, 618-641.
- U.S. Department of Health and Human Services, Food and Drug Administration. (2017, November 7). Code of Federal Regulations Title 21, Part 11: Electronic Records; Electronic Signatures. Retrieved from Food and Drug Administration: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/ cfcfr/CFRSearch.cfm?CFRPart=11.
Mark Sawicki brings 15 years of business development and sales management experience, having consistently delivered on corporate revenue and market share goals in the pharmaceutical and biotechnology industries. Sawicki was most recently the chief business officer at AAIPharma Services Corporation/Cambridge Major Laboratories Inc.
Additionally, he has served in senior business development roles at CMC Biologics and Albany Molecular Research Inc. (AMRI), where he increased revenue at rates far outpacing industry standards. Sawicki holds a bachelor’s in biochemistry from the State University of New York at Buffalo and a Ph.D. in biochemistry from the State University of New York at Buffalo, School of Medicine and Biomedical Sciences. He also received graduate training at the Hauptman Woodard Medical Research Institute. Sawicki has authored a dozen scientific publications in drug discovery with a focus on oncology and immunology.Mark W. Sawicki, Ph.D., Chief Commercial Officer