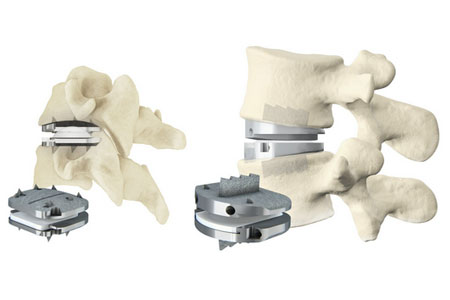
Centinel Spine®, LLC, ("the Company") a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), will present at the 2023 Castellvi Spine Symposium.
Ed McShane, Head of Motion Preservation Product Development at Centinel Spine, will provide an overview of the present and future of TDR technologies, including keys to successful device designs and pathways for future development. McShane was instrumental in the 2022 U.S. launch of the prodisc C Vivo and prodisc C SK cervical TDR system, which are the most recent TDR technologies to be approved by the U.S. Food and Drug Administration. These new TDR devices, along with the existing prodisc C, make up the Match the Disc™ system, an innovation that sets prodisc apart from all other TDRs, worldwide.
According to James Billys, MD, Orthopedic Spine Surgeon at Florida Advanced Spine and Orthopedics in Tampa, Florida and Castellvi Spine Symposium Course Chair, "Adding a technical expert to our faculty of presenters will provide excellent insight into some of the key current and future design challenges related to total disc replacement implants.
This type of insight supports clinical advancement for this procedure, as surgeons constantly look to improve patient outcomes. Centinel Spine is certainly one of the industry leaders and innovators in total disc replacement, and I am grateful for the support in adding the top engineer from this company to our clinical program."
"With the introduction of prodisc C Vivo, SK, and Nova, to complement prodisc C, Centinel Spine has continued to innovate and evolve the disc replacement options available to spine surgeons in the U.S.," said Centinel Spine CEO Steve Murray. "We are honored that Ed McShane has been selected to present at the Castellvi meeting; as the lead engineer at Centinel Spine bringing the next generation of disc replacement innovations to the market, Ed is uniquely qualified to speak to TDR evolutions and future challenges & opportunities in the space."
Centinel Spine's prodisc portfolio represents the most comprehensive TDR systems in the world, offering both cervical and lumbar implants with anatomically-differentiated designs. Centinel Spine is the only company with FDA approval for both cervical and lumbar TDR systems. All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 240,000 implantations worldwide.*
For more information, please visit www.CentinelSpine.com