Are we prescribing antibiotics wrong?

Outsourcing Clinical Trials Outside of the US

Regulators And Standards Groups Take Steps To Address Emerging Technologies In Biopharma

Two-dimensional liquid chromatography with multiple heart-cutting
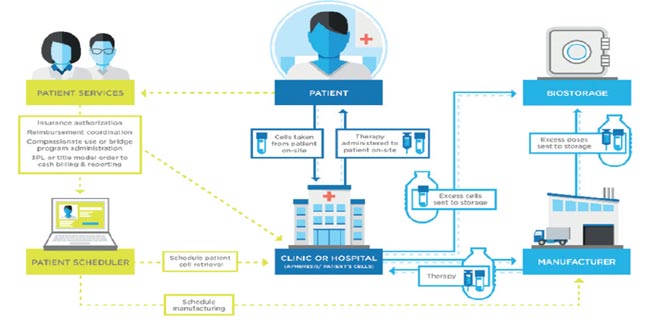
Understanding Pharmaceutical Logistics Validation in a Dynamic Environment





Latest White Papers
- Random
Featured News
Latest News
- Title
- Most Views
- Recently Added
- Ordering
- Random



Acute liver injury (ALI), often triggered by conditions such as acetaminophen overdose, can rapidly ...

BD a leading global medical technology company, and Ypsomed, a leading developer of injection ...

Biohaven Ltd. a global clinical-stage biopharmaceutical company focused on the discovery, ...

AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and ...



Latest Updates
Johnson & Johnson a worldwide leader in multiple myeloma therapies, announced that the U.S. Food and Drug Administration (FDA) approved TECVAYLI® plus DARZALEX FASPRO® (daratumumab and ...
Johnson & Johnson a worldwide leader in multiple myeloma therapies, announced that the U.S. Food and Drug Administration (FDA) approved TECVAYLI® plus DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) for the treatment of adults with relapsed or refractory multiple myeloma (RRMM) who have ...
BD a leading global medical technology company, announced it has obtained CE Marking for the Revello™ Vascular Covered Stent, a next-generation endovascular solution for the treatment of ...
BD a leading global medical technology company, announced it has obtained CE Marking for the Revello™ Vascular Covered Stent, a next-generation endovascular solution for the treatment of atherosclerotic lesions in the common and external iliac arteries.
This advancement arrives at a pivotal ...
Parnell announced the launch of Pentobarbital Sodium and Phenytoin Sodium Euthanasia Solution, a Class III controlled veterinary product formulated for humane, painless, and rapid euthanasia of dogs, ...
Parnell announced the launch of Pentobarbital Sodium and Phenytoin Sodium Euthanasia Solution, a Class III controlled veterinary product formulated for humane, painless, and rapid euthanasia of dogs, available in 100 mL multi‑dose vials.
Designed for clinical reliability and ease of ...
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, announced the launch of Diphenhydramine Hydrochloride Injection, USP in the United States.
The ...
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, announced the launch of Diphenhydramine Hydrochloride Injection, USP in the United States.
The product Is the generic equivalent of BENADRYL®, as approved by the U.S. Food and Drug Administration, ...
Otsuka Medical Devices Co., Ltd. and Otsuka Pharmaceutical Co., Ltd. (Otsuka Pharmaceutical) announce that the Paradise™ Ultrasound Renal Denervation (uRDN) system is covered by National Health ...
Otsuka Medical Devices Co., Ltd. and Otsuka Pharmaceutical Co., Ltd. (Otsuka Pharmaceutical) announce that the Paradise™ Ultrasound Renal Denervation (uRDN) system is covered by National Health Insurance system in Japan, effective March 1. Following the inception of insurance coverage, Otsuka ...
Vetter, one of the world's leading pharmaceutical service providers for the production of injectable drugs, has confirmed its plans to build a state-of-the-art production facility in the Saarland ...
Vetter, one of the world's leading pharmaceutical service providers for the production of injectable drugs, has confirmed its plans to build a state-of-the-art production facility in the Saarland region of southwest Germany.
This strategic investment marks a significant milestone in the ...