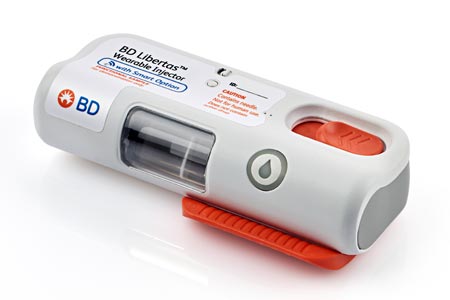
BD (Becton, Dickinson and Company), a leading global medical technology company, today announced the completion of a 50-subject human clinical trial with the BD Libertas™ Wearable Injector.
The award-winning injector* is a subcutaneous drug delivery system, currently in development, that is designed to require no patient assembly and deliver biologics with viscosities up to 50 cP in 2-5 mL and 5-10 mL configurations.
The BD independently sponsored and conducted study was designed to evaluate the performance of the 5 mL BD Libertas device in human subjects, including tissue effects, skin reactivity and patient acceptance. The results are expected to be announced early 2020.
The study represents the most recent in a series of over 50 BD conducted pre-clinical and clinical studies intended to measure the performance of the BD Libertas™ Wearable Injector, demonstrate feasibility of 2-10 mL biologic injections into subcutaneous tissue and characterize tissue response to large volume injections in human and animal subjects.
Commenting on the study, Peter Nolan, Worldwide President, BD Pharmaceutical Systems, said, “BD is committed to bringing value to our pharma partnerships, including providing them with independent BD sponsored and generated study data to accelerate combination product development. The recent study reflects BD’s continued investment in solutions to meet pharma’s needs by expanding the design space for biologics delivery.”
For more details, visit: https://www.bd.com/en-in