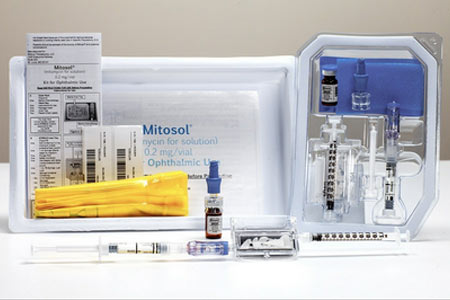
Mobius Therapeutics™, LLC, a St. Louis-based perioperative ophthalmic pharmaceutical company, has been issued US Patent Injection Apparatus and Method of Use.
This patent protects future applications of Mitosol®, Mobius' flagship product, particularly versus compounded, unapproved copies of Mitosol®.
"This is an important addition to Mobius' IP portfolio," said Ed Timm, CEO of Mobius Therapeutics. "As the gold standard for ophthalmic mitomycin, this patent acknowledges the novelty and merits inherent to Mitosol®.
It protects the apparatus itself, its unique ability to protect healthcare workers, and its ultimate flexibility in accommodating all clinical needs."
Mitosol® is the only FDA approved formulation of mitomycin-c bearing an ophthalmic indication. With room temperature storage, shelf life of up to 24 months, and USP <800> compliance, Mitosol® offers unique flexibility to providers, as its "shelf-ready" status permits on demand utility.
"Ophthalmic surgeons are smart and resourceful physicians, relentlessly advancing clinical excellence with improved clinical technique," continued Timm.
"Mobius™ shares this passion; franchise protection provides the resources required to respond with products to meet and exceed these demanding needs, now and into the future. In the end, everyone wins: the patient, the provider, and the American healthcare system."