Are we prescribing antibiotics wrong?

Outsourcing Clinical Trials Outside of the US

Regulators And Standards Groups Take Steps To Address Emerging Technologies In Biopharma

Two-dimensional liquid chromatography with multiple heart-cutting
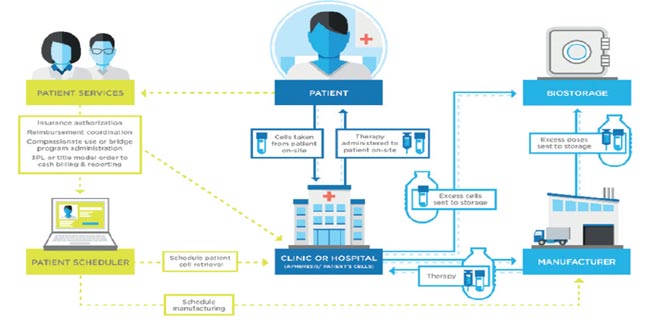
Understanding Pharmaceutical Logistics Validation in a Dynamic Environment





Latest White Papers
- Random
Featured News
Latest News
- Title
- Most Views
- Recently Added
- Ordering
- Random



BD a leading global medical technology company, and Ypsomed, a leading developer of injection ...

Biohaven Ltd. a global clinical-stage biopharmaceutical company focused on the discovery, ...

AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and ...

Virax Biolabs Group Limited an innovative biotechnology company dedicated to the advancement of ...



Latest Updates
Zinc Saves Kids, a global child‑health initiative of the International Zinc Association (IZA), has announced a new partnership with leading Zambian pharmaceutical manufacturer Pharmanova Zambia ...
Zinc Saves Kids, a global child‑health initiative of the International Zinc Association (IZA), has announced a new partnership with leading Zambian pharmaceutical manufacturer Pharmanova Zambia Limited to expand access to life‑saving zinc treatments for children across Zambia.
The collaboration ...
SurGenTec®, a medical device innovation company focused on advancing treatment options for orthopedic and spine surgery, announced FDA clearance of its ION-C™ Facet Fixation System.
ION-C™, part ...
SurGenTec®, a medical device innovation company focused on advancing treatment options for orthopedic and spine surgery, announced FDA clearance of its ION-C™ Facet Fixation System.
ION-C™, part of SurGenTec®’s posterior cervical platform, has received expanded indications allowing for the ...
PICO IV, a leading innovator in sterile cannabidiol (CBD) solutions, announced a closely coordinated clinical collaboration with BrainThrive, PC, a neurology and diagnostic testing center in Westlake ...
PICO IV, a leading innovator in sterile cannabidiol (CBD) solutions, announced a closely coordinated clinical collaboration with BrainThrive, PC, a neurology and diagnostic testing center in Westlake Village, California.
The partnership places PICO IV's patented sterile CBD solution within a ...
AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and protection technologies, announced that its innovative nasal vaccine delivery solutions, LuerVax® and ...
AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and protection technologies, announced that its innovative nasal vaccine delivery solutions, LuerVax® and Spray Divider™, are being utilized in CastleVax’s Phase II clinical trial of CVAX-01, a next-generation ...
Fabentech, a French biopharmaceutical company specializing in medical countermeasures against biological threats, announces that it has been granted Marketing Authorization for Ricimed®, a treatment ...
Fabentech, a French biopharmaceutical company specializing in medical countermeasures against biological threats, announces that it has been granted Marketing Authorization for Ricimed®, a treatment for ricin poisoning.
Ricimed®, an antidote against one of the most toxic natural substances in ...
Dechra, a global leader in veterinary specialty care, today shares the FDA approval of Emeprev™ (maropitant citrate) Injectable Solution, the first FDA-approved bioequivalent injectable solution to ...
Dechra, a global leader in veterinary specialty care, today shares the FDA approval of Emeprev™ (maropitant citrate) Injectable Solution, the first FDA-approved bioequivalent injectable solution to the most widely used antiemetic for dogs and cats.
Indicated for the prevention and treatment of ...